
How Artificial Intelligence and Telemedicine are Transforming Hybrid and Home-Based Trials
Digitalization and telemedicine have shown significant advancements in the medical industry. Still, clinical research will continue to need high-quality tools to conduct trials efficiently and decrease strain on patients.
Since the Covid-19 pandemic started over two years ago, it has led to great advances in healthcare and medicine, notably the first-ever mRNA vaccine against Sars-CoV-2 in 2020. Initially out of necessity, now with the potential to support and enhance the healthcare system, a surge in demand for digitalization and new digital systems have emerged, for instance, to conduct PCR tests digitally from home and drop off the sample at a nearby grocery store or gas station. It is evident that innovative digital solutions are transforming the healthcare sector, including clinical research, by significantly increasing the efficiency of trial protocols and high-quality results while also enabling patient centricity via electronic Patient Reported Outcomes (ePROs). There is no doubt that the healthcare industry has just begun utilizing the full potential of digitalization and technology.
Improvement of clinical trials via remote patient monitoring
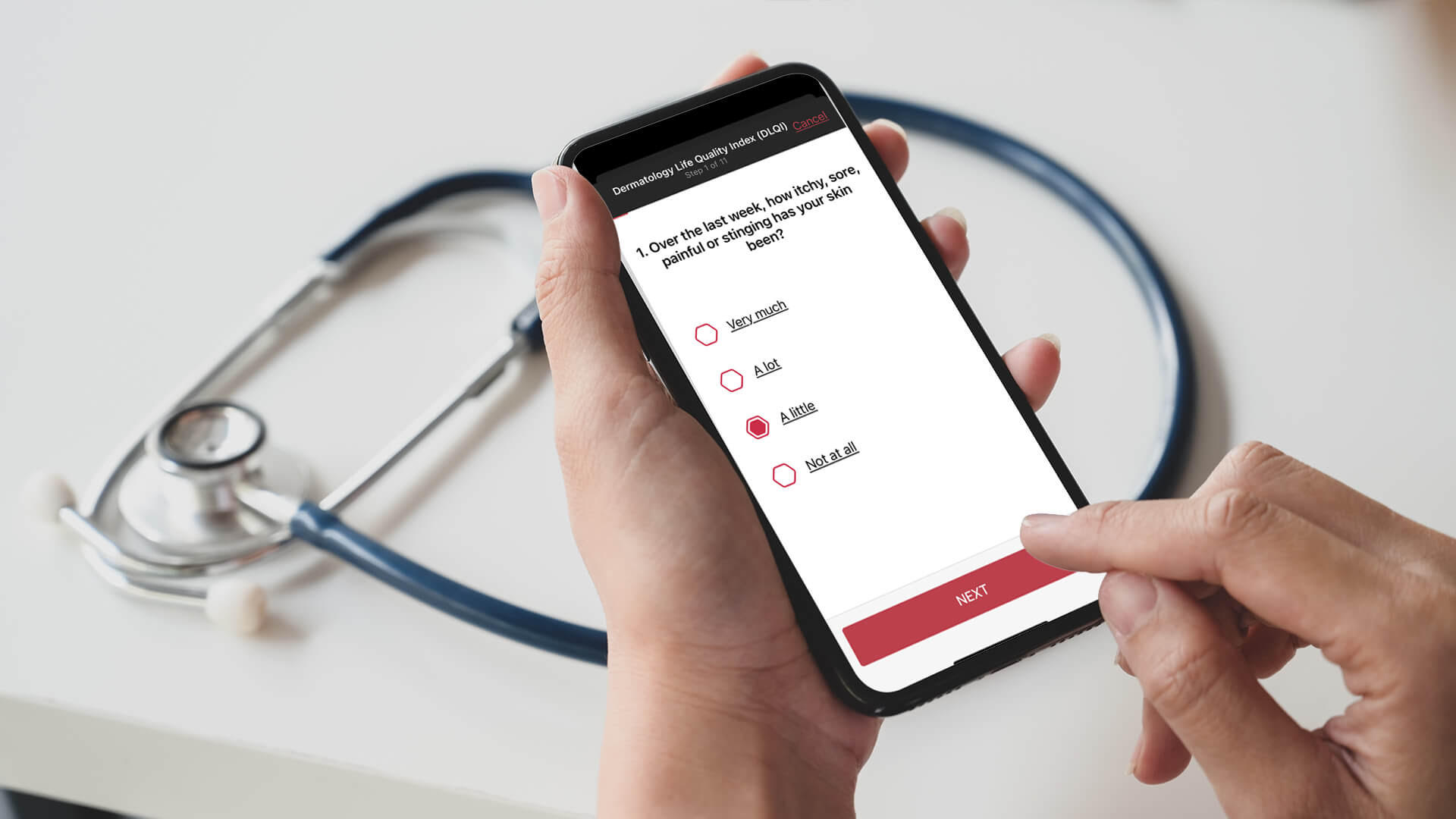
In the last two years, we have seen a rise in demand for telemedicine options, not only for regular medical check-ups that do not require physical examinations but also for medical research, namely clinical trials. In traditional clinical trials, participants are required to attend regular in-person checkups or receive treatments at the defined clinic or hospital. Hybrid or home-based trials provide additional patient safety compared to a completely centralized trial. Especially in the current situation, it can prevent clinical trial subjects from becoming infected with Sars-CoV-2 during their hospital visit or possibly infect others. If in-person appointments are not mandatory for every session, hybrid trials can help create a more efficient environment, saving time and reducing costs while keeping a high standard of care. The virtualization of some aspects of trials allows the medical staff to recruit patients, obtain informed consent and monitor safety efficiently. These implementations could benefit medical research, pharmaceutical, and biotechnology companies. To ensure that digital patient data is safely stored, a medical tool and digital platform must also meet the high standards of data privacy regulation.

How Scarletred®Vision can enhance efficiency and results in clinical study phases I-IV
Incorporating digital systems at an early research stage provide consistent and high-quality data. Based on artificial intelligence (AI) and machine learning, our digital solution Scarletred®Vision provides standardized images and offers tools to analyze skin changes in over 3000 dermatological diseases by taking a photo of the affected area with the Scarletred®Vision skin patch. This innovative CE-certified class I medical device provides efficient diagnostic support for the medical staff. Our goal is to understand the specific study requirements, customize our services accordingly, and implement them in a smaller environment. The software can be entirely customized as a flexible SaaS (Software as a Subscription) based on the specific needs and requirements of the clinical trials. The early adoption of Scarletred®Vision can provide mutual benefits as our tool offers added value to a project by generating quantifiable smart data, reducing costs, and significantly improving the quality and outcome of the research study. However, the software is also very beneficial for phase IV post-marketing studies. The information and patient data are sent directly via the app to the study sites for central reading, meaning the study sites employ the same methods and protocols for the investigations, so standardized data is essential. Clinical phase IV studies require drug monitoring also after approval to assure long-term safety and effectiveness of the medication or pharmaceutical. Scarletred®Vision can therefore be used to track patient responses, effect on the quality of life, and possible side effects of the drug conveniently for the patient, and the trial coordinators receive useful smart data, not big data.

Pushing the limits of clinical trials with AI
Implementing AI and deep learning algorithms can elevate and simplify decentralized trials. SCARLETRED’s clinically validated skin imaging and analysis tool allows to push the limits of clinical research and provide a digital solution for efficient data collection as diagnostic support for medical staff. Digitalization in healthcare and the medical field will help enhance healthcare services and future clinical research, which requires a safe environment and high-quality data. This will become the next generation of decentralized clinical research.



